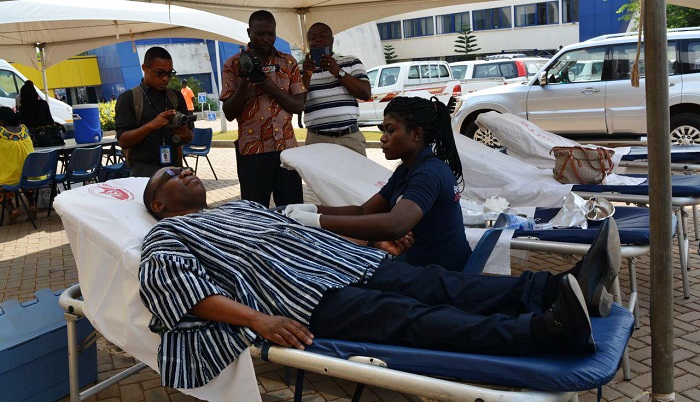
FDA to regulate blood collection to ensure safety
The Food and Drugs Authority (FDA) has developed guidelines to regulate the collection of blood and blood components used for transfusion.
Advertisement
According to the FDA, the decision to regulate blood and its derivatives was in line with the Public Health Act of 2012, Act 851.
The law defined therapeutic products to include blood and blood products which must have standards regulating them to protect public health.
The authority is thus seeking to use the guidelines to progressively ensure protection of patients from unsuitable blood and blood products.
Under the guidelines, the FDA would enforce quality standards through the inspection of blood establishments, monitoring of accidents and adverse clinical events should they arise.
Event
Speaking to the Daily Graphic on the sidelines of a blood donation exercise organised by the FDA in Accra, the Chief Executive Officer (CEO) of the FDA, Mr Hudu Mogtari, said the World Health Organisation (WHO) had issued a resolution requiring that blood be considered as a therapeutic agent for which reason its handling and preparations must be governed by certain standards and rules.
In this regard, he indicated that the FDA had signed a Memorandum of Understanding (MoU) with the National Blood Service for the regulation of the processes used by the service in order that it puts in measures to ensure that end blood products were safe for use.
Under the MoU, the FDA would inspect all blood establishments (facilities) periodically to ensure quality standards.
“Blood in certain jurisdictions is prepared differently with component often separated for specific uses, hence the FDA would ensure that the methods used for collection and the environment in which it is prepared must be safe, while personnel involved in the process must have the requisite qualification and knowledge,” Mr Mogtari said.
Risks
The Head of the Biological Unit of the FDA, Dr Edwin Nkansah, said biological products such as blood and blood products were likely to come along with some inherent risk of infectious agents.
He said even though a zero risk was unattainable, the FDA could drive such risks to the lowest level achievable without unduly decreasing the availability of blood as a life-saving resource.
“The WHO has listed blood and its derivatives as parts of biological products which are essential medicines that must be of good quality and affordable”, Dr Nkansah said.
Writer’s email: [email protected]




