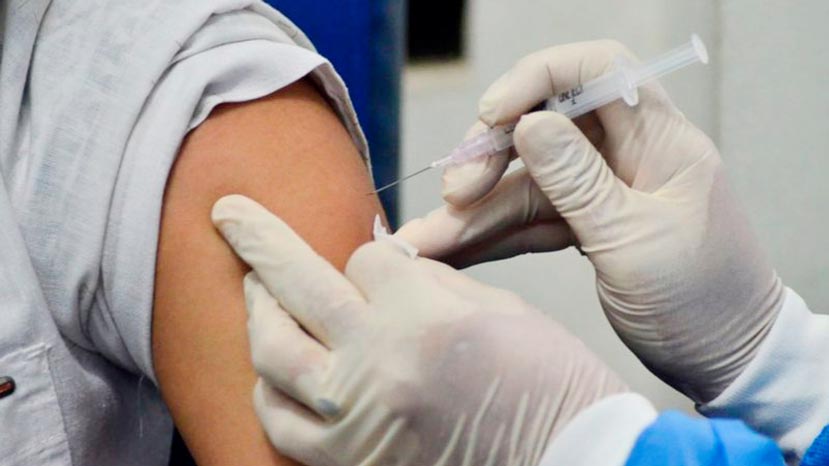
COVID-19 vaccine... waiting with bated breath
It has been almost a year since the new coronavirus plagued the world. As of October 20, 2020, there have been 40.4 million cases worldwide with 1.12 million deaths. Ghana has recorded 47,372 cases with 310 deaths.
The virus affects people differently and people who have contracted the disease have been treated according to the symptoms they exhibit.
Advertisement
However, with an increasing number of cases, doctors now know that the loss of smell and the loss of taste are classical symptoms of COVID-19, with other less classical symptoms being fever, coughing and sneezing.
As the influenza season in the northern hemisphere approaches with winter, several countries will be giving vaccines to vulnerable people, including the aged to prevent seasonal influenza.
However, there is no vaccine against COVID-19, with its catastrophic destruction of the global economy. The whole world is eagerly waiting for a vaccine that will protect the body against the disease and eventually lead to total eradication of COVID-19. That appears to be humanity’s best chance of fighting and winning the war against COVID-19.

PHOTO: There are more than 100 candidate vaccines under various stages of development
Importance vaccine
The Director-General of the Ghana Standards Authority (GSA), Prof. Alex Dodoo, notes that a vaccine is needed because vaccines prepare the body’s own immune system to identify, and fight off diseases caused by bacteria or viruses.
A COVID-19 vaccine, he explains, will, therefore, prepare recipients to fight off any COVID-19 infection and disease. Currently, immunisation prevents up to three million vaccine-preventable deaths each year around the world.
Prof. Dodoo says vaccines are critically important because vaccination helps prevent infection. With a reduction in infection, there will be very few viruses being passed on.
“Where this is successful, diseases caused by the virus disappears and the virus is eventually eliminated and eradicated. This has occurred with smallpox and can happen with COVID-19,” he says.
Promise
Currently, there are more than 100 candidate vaccines under various stages of development. As of the beginning of October 2020, 92 of these vaccines were in what is called “pre-clinical development,” meaning they are yet to be given to any human being.
In addition to these, there are 44 vaccines which have been demonstrated to be safe for humans and have also shown promise and are thus being tested in human beings – that is, they are undergoing clinical trials.
Prof. Dodoo says trials of vaccines occur typically in phases with Phase 1 being the testing of the vaccines in a limited number of people to see whether they are safe and the dose to give.
Phase 2 trials also examine safety, but also see whether the vaccine actually works in a slightly bigger number of people compared to phase 3. Phase 3 trials examine the safety and efficacy of the vaccines in large numbers of people.
Where there is an urgent need for vaccines and when the vaccine has also shown promise, it is possible to grant approval for limited use among the population or identified groups in the population.
Currently, there are five vaccines out of the 11 in phase 3 clinical trials which have been granted authorisation for limited use. These are COVID-19 vaccines by CanSino BIO (China), Gamaleya Research Institute (Russia), Wuhan Institute of Biological Products (China), Sinopharm (China) and Sinovac Biotech (China).
It is expected that at least two or more other vaccines in clinical trials will be ready for widespread use worldwide, including those developed by AstraZeneca and Oxford University (UK), Johnson & Johnson (US) and Moderna in collaboration with the National Institutes of Health (US).

PHOTO: The trial of the OxfordAstraZeneca vaccine is at an advanced stage.
Trials
African countries have remarkably been absent in the clinical trials of these new vaccines. There have been conspiracy theories which have led to fear and uncertainty among the population leading to inertia.
However, Prof. Dodoo says South Africa stands out as the exception on the continent as it has been active in the clinical trials of some COVID-19 vaccines.
Countries which do not take part in vaccines lose out as the new products will not have been tried in their population thereby making it difficult to know how well these vaccines will work in those countries.
For Prof. Fred Binka of the University of Health and Allied Sciences (UHAS), Ho, he thinks it is crucial that the vaccine is tested in the population that it is going to be used.
He says the basics of science in the development of a vaccine and the mechanism in which the antigens react in different species make it necessary for the vaccine to be tested in our population.
Prof. Binka said the antigens in COVID-19, to which the vaccine was being directed, might be different in different species
“So if America is developing a vaccine against COVID-19, it is for the strain in America; if Europe is developing a vaccine, it is for the strain in Europe and if China is developing a vaccine, it is for the strain in China”.
He says so far, there is no evidence to show that the vaccine being produced has cross-protection because it is a new strain of virus.
Africa, COVAX facility
As of October 1, 167 countries had signed to join the COVAX facility, a mechanism designed to guarantee rapid, fair and equitable access to COVID-19 vaccines worldwide.
The CEO of Gavi, the Vaccine Alliance, Dr Seth Berkley, says COVAX is the only truly global solution to this pandemic because it is the only effort to ensure that people in all corners of the world get access to COVID-19 vaccines once they are available, regardless of their wealth.
But Africa has cause to worry. In an article titled “COVID-19 vaccines: How to ensure Africa has access”, published in Nature, the authors (John N. Nkengasong et al) note that already several high-income countries have signed their own contracts with individual companies to buy selected vaccines.
For instance, the article said the United States had made deals worth upwards of $6 billion with several firms.
Additionally, an analysis by the international charity, Oxfam, finds that even if all five of the most advanced vaccine candidates succeed, there will not be enough vaccine for most of the world’s people until 2022.

PHOTO: British pharma giant AstraZeneca and Oxford University are developing a coronavirus vaccine
When?
Prof. Dodoo is optimistic there would be a COVID-19 vaccine soon. “There will surely be several COVID-19 vaccines for widespread use by the end of 2020. Widespread availability will most likely be towards the middle of 2021.
However, efficacy of these vaccines is likely to be variable and it will probably take between 12 and 24 months to identify those vaccines that are highly effective and safe for widespread distribution and use. By that time, COVID-19 may already be waning due to mutations or just natural causes,” he says.
Writer’s Email: [email protected]



